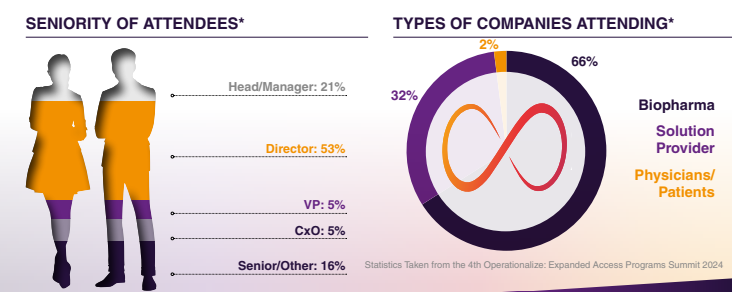
About Event
The 5th Operationalize: Expanded Access Programs Summit was the the premier event for connecting expanded access professionals with their industry peers to share proven strategies for overcoming operational and regulatory barriers, ensuring access to life-changing therapies outside traditional clinical trials.
Featuring deep-dive discussions and actionable case studies, the Summit addressed global regulatory differences, best practices for transitioning patients to commercial programs, and how to leverage data to support regulatory filings, clinical trials, and therapeutic development. This was the must-attend event for experts aiming to elevate their Expanded Access Programs in 2025 and beyond.
In March 2025, your peers joined at this essential gathering, where the brightest minds in Expanded Access from companies like Novartis, Pfizer, BMS, Biogen, Ascendis, and others converged. This collaborative, non-competitive event was designed to equip you with the knowledge and tools needed to bridge the gap between cutting-edge clinical research and the patients who need it most.
What You Missed in 2025:
Participating in an interactive, two-track workshop day, offering actionable insights for the entire Expanded Access community, regardless of experience level. Workshops are hosted by Daiichii Sankyo, Blueprint Medicines, Gilead Sciences, Pfizer, BeiGene, Takeda, and EMD Serono.
Turbocharging your understanding of country-specific regulatory requirements to successfully run compliant global Access Programs, with expert insights from EMD Serono, Shionogi, Novartis, and The Max Foundation.
Gaining insights into the operational challenges of running Access Programs in low-to-middle-income countries, ensuring equitable patient access, with guidance from GARDaccess.
Navigating the regulatory complexities of transitioning patients from free-of-charge to commercial programs, ensuring sustainable continuity of care, with insights from Blueprint Medicines.
Exploring an innovative funding model that generates actionable insights, supplements clinical trial data, and informs regulatory decisions, led by Biomed Valley Discoveries.
Who Was There?